Cardiovascular Section
The information on this page is intended for health professionals reference and use only. If you are a parent or family member looking for information about treatment, please visit unit information.
Contents
1.0 Aim of guideline
2.0 Scope of guideline
3.0 Guideline
Appendix 1 – Echocardiographic and clinical scoring
Appendix 2 – PDA Ligation Referral Pathway
Appendix 3 – PDA Ligation Referral Pathway – Guidance Notes
Appendix 4 – PDA Ligation Referral Form
Appendix 5 – PDA Parent Information Leaflet
Appendix 6 – Paracetamol administration details, PDA closure, monitoring and further information
Appendix 7 – Ibuprofen administration details, monitoring, further information and repeated course
1.0 Aim of guideline
A guideline framework to support clinicians in the management of Patent Ductus Arteriosus (PDA) in neonates within neonatal services within TV & Wessex Neonatal ODN. Medical management is only indicated in the presence of one or more definite clinical symptoms and ECHO evidence of haemodynamically significant PDA.
2.0 Scope of guideline
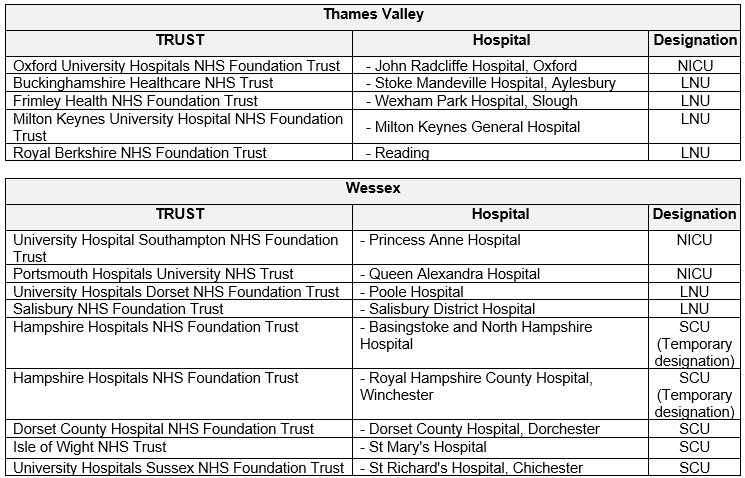
This guideline applies to all neonatal Units within the TV & Wessex Neonatal ODN. This includes the following hospitals.
3.0 Guideline
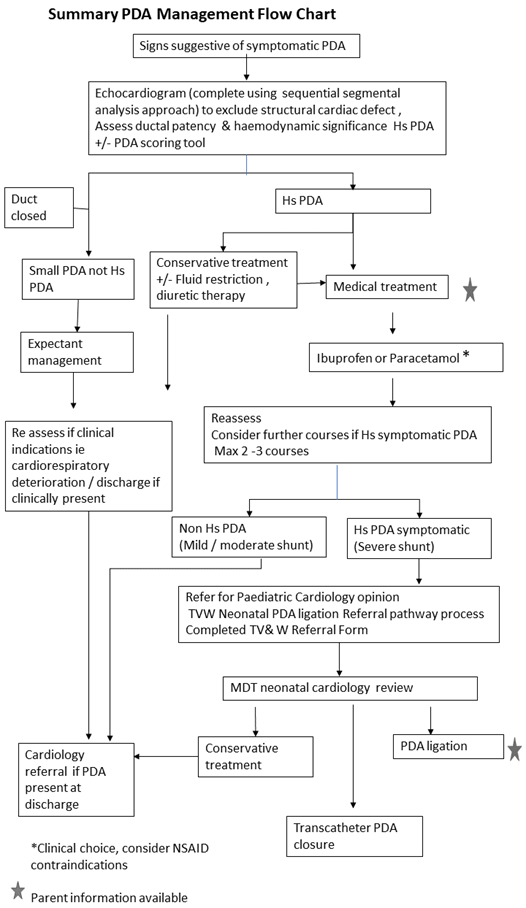
Background, treatment options and definition of ‘symptomatic’ treatment
There is a wide variation in the rate of spontaneous ductal closure in preterm babies. A persistently patent duct with a large ductal shunt can lead to increased pulmonary perfusion, as well as decreased systemic blood flow (and/or end-organ perfusion); and has been associated with increased mortality and numerous preterm morbidities (fluctuations in BP and resultant IVH, pulmonary haemorrhage, ventilator dependence, CLD and NEC).
Pharmacologic treatment with COX-inhibitors, cyclo-oxygenase inhibitors (ie indomethacin, ibuprofen) and paracetamol) can be prophylactic, ‘pre-symptomatic’ (before the evolution of symptoms of ductal shunt) or ‘symptomatic’ (treatment is delayed until the presence of clinical symptoms). Prophylactic treatment with indomethacin or ibuprofen hasn’t been associated with increased survival or better long-term outcome. There is not enough evidence at present to recommend prophylactic paracetamol administration. Recent results from RCTs comparing echo screening and early pharmacologic treatment in asymptomatic babies versus delaying treatment until well-defined clinical symptoms of high-volume ductal shunt were met demonstrated no benefit (or very little benefit (TRIOCAPI)) of echo screening.
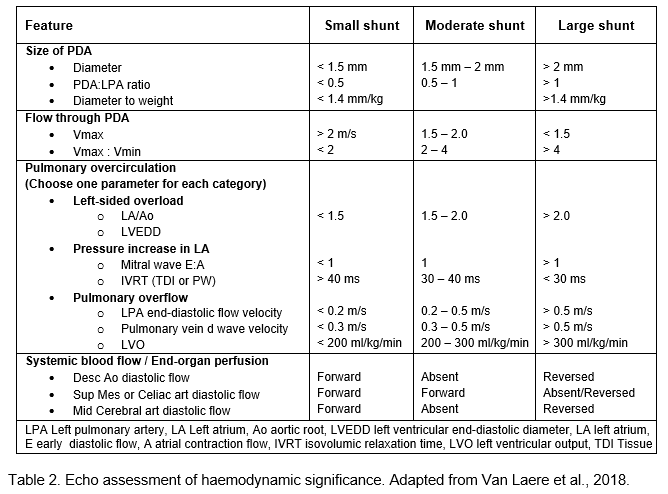
In view of these results, routine echo screening and pharmacologic treatment in preterm infants without clinical symptoms are not indicated. Preterm and IUGR infants below 32 weeks with clinical symptoms (as below) should undergo diagnostic echocardiography and receive treatment if needed. Scoring systems for clinical symptoms and echo findings of haemodynamic significance have been introduced recently to aid decision-making. An example is shown in Appendix 1.
Clinical symptoms suggestive of high-volume ductal shunt:
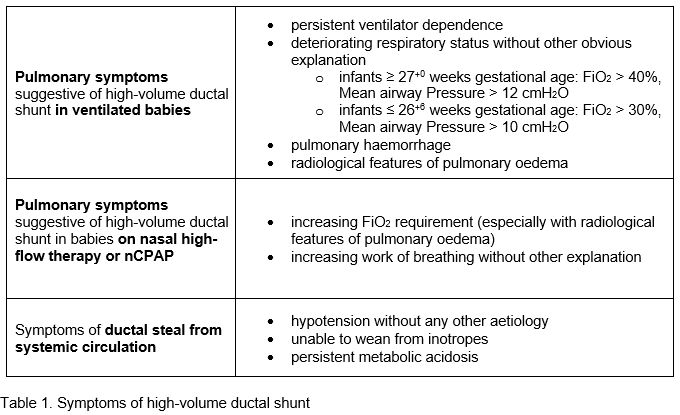
Echo features of haemodynamic significance. Initial assessment should include establishment of normal structure (complete sequential segmental analysis). If baby doesn’t tolerate study, please measure PDA size, flow pattern, LA:Ao, LPA end-diastolic flow velocity and flow pattern in descending aorta.
Conservative management:
Fluid restriction Consider fluid restriction (not below 120 ml/kg/day) and diuretic therapy. Chlorothiazide and spironolactone are preferred over furosemide if enteral intake is more than 50% of total intake. Once full enteral feeding has been established, fluid intake can be increased in order to reach adequate energy and protein intake with the concurrent administration of diuretics.
PEEP Consider increasing PEEP by 1-2 cm H2O if ventilated or on nCPAP. No data on nHFT.
Correct anaemia Keep Hb > 12 g/L in ventilated preterm infants with significant PDA. Transfuse with co-administration of diuretics ie Furosemide.
Pharmacological treatment:
Pharmacological treatment is only indicated in babies with PDA-related clinical symptoms and echo-confirmed haemodynamically significant PDA. Use of COX-inhibitor in asymptomatic babies is not indicated, unless very significant echo findings. Scoring systems for clinical and echo findings can aid decision-making. An example is shown in Appendix 1.
Ibuprofen or Paracetamol
Recent meta-analyses suggest that the efficacy of Paracetamol and Ibuprofen is very similar, but Paracetamol has a more favourable side effect profile (less NEC, GI bleeding and renal impairment). Most studies included very preterm babies (GA < 32 weeks) and there is a relative paucity of data regarding the more preterm population (GA < 28 weeks). Well-designed studies in babies GA < 28 weeks are pending. It is difficult to make firm recommendation at present which drug should be used as routine first-line.
High-dose Ibuprofen (beyond day 7) has been reported to be more effective than standard dose. Oral administration is more effective for both drugs.
Use standard dose Ibuprofen 3 day course: 10mg/kg/OD on day 1 followed by 5mg/kg/OD on days 2 and 3 at 24 hourly intervals or Paracetamol (15 mg/kg, QDS, for 3 days) as routine pharmacological treatment.
Use oral preparations (rather than IV) if baby is receiving >100 ml/kg enteral feeds; the use of oral preparations can be considered with smaller enteral volumes (senior clinician choice) Re-assess the ductus arteriosus and ductal shunt after 3 days;
A second course or course extension to 6 days of high dose Ibuprofen (3 day course: 10mg/kg/OD on day 1 followed by 5mg/kg/OD on days 2 and 3) or same dose Paracetamol can be considered, if necessary. A third course of the alternate drug might be considered, but literature data about the efficacy of a third course is scarce.
Contraindications
Ibuprofen Life-threatening infection; active bleeding; thrombocytopenia (platelets <50); coagulation defects; significant renal impairment; known/suspected NEC; pulmonary hypertension; duct-dependent circulation, recent IVH (within 24 hours).
Side effects GI perforation (consider Ibuprofen carefully in IUGR and after hydrocortisone administration due to pressor-resistant hypotension); increased serum creatinine; hyponatraemia; oliguria; fluid retention; acute renal failure, platelet dysfunction and thrombocytopenia; neutropenia; haematuria; pulmonary haemorrhage; IVH; PVL. Less common: GI haemorrhage; hypoxaemia.
Monitor/Caution Watch for signs of bleeding; may mask symptoms of infection; monitor renal function. Ibuprofen may decrease clearance of aminoglycosides so strict surveillance of serum levels is recommended. In cases of oliguria or rising creatinine, doses of aminoglycosides should be held until levels are available. Ibuprofen interferes with bilirubin-albumin binding increasing unbound bilirubin and should not be used in infants with hyperbilirubinaemia approaching exchange transfusion levels.
Paracetamol Use Paracetamol if there are contraindications to Ibuprofen. Overdose can cause liver toxicity. Check liver function before each course and at least once during the course.
Ligation:
PDA ligation should not be the primary treatment of choice and should be preceded by pharmacological treatment where possible. Ligation is generally not recommended in the first 3 weeks of life. If multiple courses of medical treatment have failed to close or restrict a haemodynamically significant duct and echo assessment confirms a high-volume shunt in a symptomatic patient refer for a paediatric cardiology opinion. If respiratory problems are predominant consideration of steroids and diuretics should be given prior to PDA ligation.
Ligation is only indicated when a) the PDA echo score is high (haemodynamically significant duct on echo) and b) there is no other explanation for persistent clinical symptoms such as:
- Increasing ventilatory requirement over several days (FiO2 > 40-50%, MAP >12-13 cmH2O) with signs of pulmonary hyperaemia on the CXR or ventilator dependence and unable to extubate.
- Hypotension requiring inotropic support.
- Oliguria/renal failure.
Transcatheter PDA closure
Although transcatheter closure of PDA is common practice in older infants and children, it is still a relatively new approach in preterm infants. In this method, a device is used to plug the PDA via a transcatheter approach through the femoral vein. The data although limited is encouraging. However more studies are needed for analysing long term and short-term outcomes compared to surgical ligation.
In selected cases of preterm infants of reasonable size (body weight approximately 2 kg and more), this option for PDA closure could be considered with the cardiology team. This should include a clear plan around transport to the theatres, temperature management and support in the theatre and the logistics of the post-operative recovery period.
Appendix 1.
Echocardiographic and clinical scoring (adapted from Brigham’s Neonatal Unit, Boston, MA guideline)
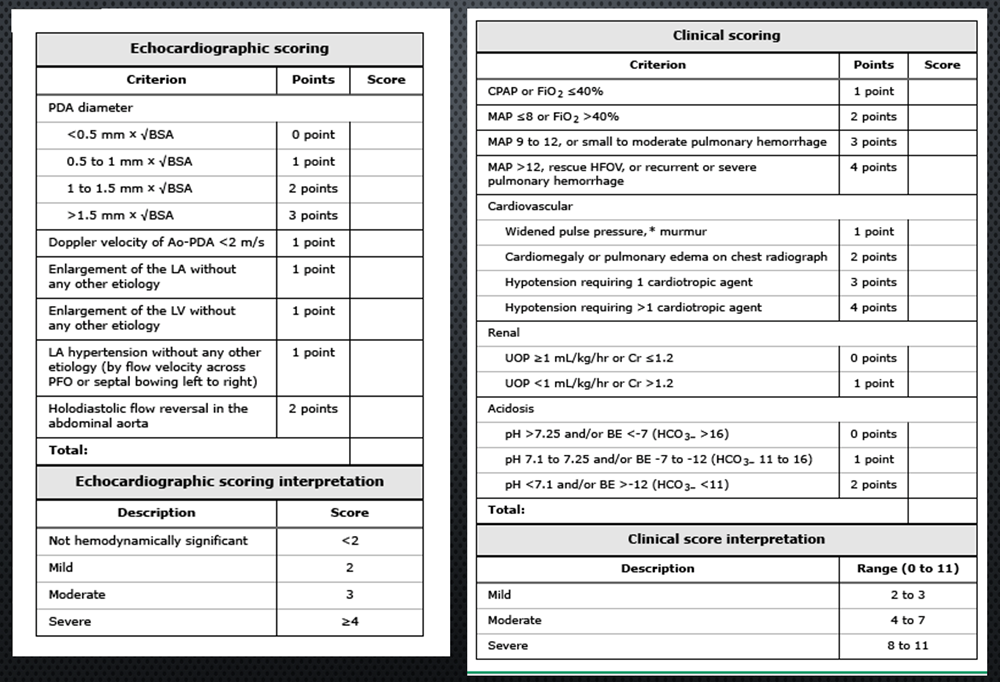
Please click on the link below to calculate the PDA score on the adapted PDA SCAMP tool
Appendix 2.
PDA Ligation Referral Pathway (from TVW Neonatal ODN Cardiac Care Pathway v1.3)
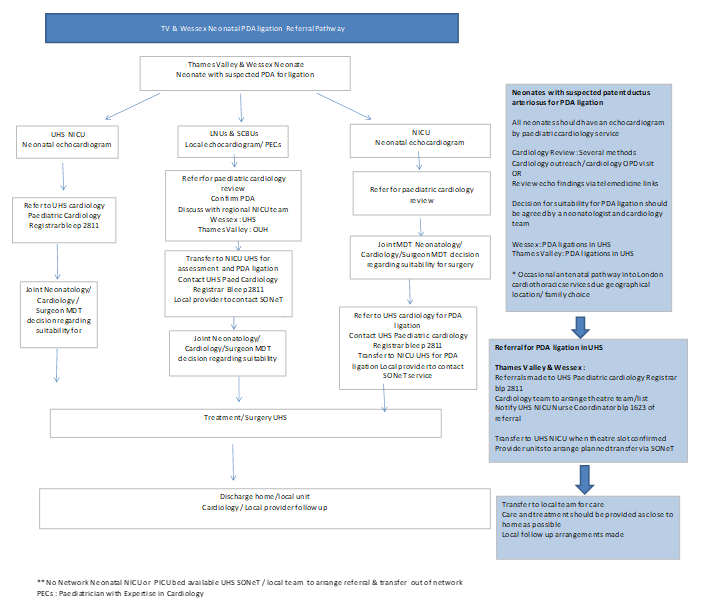
Appendix 3.
Appendix 4.
Appendix 5.
Version control:
| Version | Date | Details | Author(s) | Comments |
| Final v1 | 14 Sep ‘15 | OUH Guideline for Thames Valley Network | Dr Zoltan Molnar | Approved by Neonatal Consultants and Paediatric Cardiologists |
| Version 2 | 23 Feb ‘16 | TV Neonatal ODN Format | Dr Zoltan Molnar | Approved by TV&W Neonatal ODN Governance Group 28 April 2016 subject to agreed amendments |
| May 2016 | Amendments completed | |||
| Version 3
3.1 4
|
January 2023
April 2023 |
TV & Wessex Guidance
Update and revision of guidance |
TV & W PDA working group (ZM, RB, HH, VP, HW) | |
| Version 5 | Feb 2024 | PDA Task and Finish group revision of guidance with UHS cardiology and Suzannah Hibberd (Pharmacy) .
Circulated to TV& W Paed Cardiac Network |
TV & W PDA working group as above and SH | Comments from TB,
UHS Cardiology Lead T Richens Intervention Cardiac Lead AC , SA TV & W Paed Cardiac Network. Ratified March 2024 |
| Review Date: | March 2027 | |||
Document version
Version 6
Lead Authors
Dr Zoltan Molnar, Neonatal Consultant, OUH
TV & Wessex PDA Working Group (V Puddy, R Banerjee, H Hu, Z Molnar, H Wilson)
UHS Pharmacy (S Hibberd)
Approved by
Thames Valley and Wessex Neonatal Network Governance Group
Approved on
21 March 2024
Renew date
March 2027
Full guide
Related documents and references
Thames Valley and Wessex Neonatal Network Cardiac Care Pathway
https://neonatalnetworkssoutheast.nhs.uk/professionals/guidelines/tvw-guidelines/cardiac-care-pathway/
Thames Valley and Wessex Neonatal Network Patent Ductus Arteriosus Ligation Referral Pathway (Appendix 2)
References:
- Mitra S, de Boode WP, Weisz DE, Shah PS. Interventions for patent ductus arteriosus (PDA) in preterm infants: an overview of Cochrane Systematic Reviews Cochrane Database Syst Rev. 2023 Apr 11;4(4):CD013588
- Jasani B, Mitra S, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. 2022 Dec 15;12:CD010061. Review.
- Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the Patent Ductus Arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014 Mar; 99(2): F99-F104.
- Rozé JC et al. Effect of Early Targeted Treatment of Ductus Arteriosus with Ibuprofen on Survival Without Cerebral Palsy at 2 Years in Infants with Extreme Prematurity: A Randomized Clinical Trial. J Pediatr. 2021 Jun;233: 33-42.
- Schindler T, Smyth J, Bolisetty S, Michalowski J, Mallitt KA, Singla A, Lui K. Early PARacetamol (EPAR) Trial: A Randomized Controlled Trial of Early Paracetamol to Promote Closure of the Ductus Arteriosus in Preterm Infants. Neonatology. 2021;118(3):274-281.
- Hundscheid T et al., Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N Engl J Med. N Engl J Med. 2023 Mar 16;388(11):980-990.
- Gupta S et al. Study protocol: baby-OSCAR trial: Outcome after Selective early treatment for Closure of patent ductus ARteriosus in preterm babies, a multicentre, masked, randomised placebo-controlled parallel group trial. BMC Pediatr. 2021 Feb 26;21(1):100. Preliminary results presented at PAS, Denver, 2022
- Mitra S, Scrivens A, von Kursell AM, Disher T. Early treatment versus expectant management of hemodynamically significant patent ductus arteriosus for preterm infants Review Cochrane Database Syst Rev. 2020 Dec 10;12(12) Review
- Clyman RI et al. PDA-TOLERATE (PDA: TO LEave it alone or Respond And Treat Early) Trial Investigators. PDA-TOLERATE Trial: An Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age. J Pediatr 2019 Feb; 205: 41-48.e6.
- Sung SI, Lee MH, Ahn SY, Chang YS, Park WS. Effect of Nonintervention vs Oral Ibuprofen in Patent Ductus Arteriosus in Preterm Infants: A Randomized Clinical Trial. 2020 Aug 1;174(8):755-763. JAMA Pediatr. 2020 Aug 1;174(8):755-763.
- Mitra S et al. Association of Placebo, Indomethacin, Ibuprofen, and Acetaminophen With Closure of Hemodynamically Significant Patent Ductus Arteriosus in Preterm Infants: A Systematic Review and Meta-analysis. 2018 Mar 27;319(12):1221-1238.
- Mitra S, Weisz D, Jain A, Jong G; Canadian Paediatric Society, Fetus and Newborn Committee, Drug Therapy Committee. Management of the patent ductus arteriosus in preterm infants. Paediatr Child Health 2022 27(1): 63
- Mashally S et al. Is late treatment with acetaminophen safe and effective in avoiding surgical ligation among extremely preterm neonates with persistent patent ductus arteriosus? J Perinatol. 2021 Oct;41(10):2519-2525
- Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of Patent Ductus Arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012 Jun; 160(6):929-35.
- Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the Patent Ductus Arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. 2012 May; 32(5):344-8. Acta Paediatr. 2013 Mar; 102(3):254-7.
- Rolland A, Shankar-Aguilera S, Diomandé D, Zupan-Simunek V, Boileau P. Natural evolution of Patent Ductus Arteriosus in the extremely preterm infant. Arch Dis Child Fetal Neonatal Ed. 2014 Aug 28. Epub ahead of print
- Weisz DE, McNamara PJ. J Patent Ductus Arteriosus ligation and adverse outcomes: causality or bias? J Clin Neonatol. 2014 Apr;3(2):67-75. Review
- Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of Patent Ductus Arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010 Sep; 157(3):381-7.
- Eursiriwan S et al. Comparison of Various Pharmacologic Agents in the Management of Hemodynamically Significant Patent Ductus Arteriosus in Preterm: A Network Meta-Analysis and Risk-Benefit Analysis. Biomed 2022 Oct 24;7(3):125-145.
- Katsaras DN et al. Comparative safety and efficacy of paracetamol versus non-steroidal anti-inflammatory agents in neonates with patent ductus arteriosus: A systematic review and meta-analysis of randomized controlled trials.Br J Clin Pharmacol. 2022 Jul;88(7):3078-3100.
- van Laere D, van Overmeire B, Gupta S, El-Khuffash A, Savoia M, McNamara PJ, Schwarz CE, de Boode WP; European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE). Application of NPE in the assessment of a patent ductus arteriosus. Pediatr Res. 2018 Jul;84(Suppl 1):46-56.
- Vali, P., Lakshminrusimha, S., Pelech, A. et al.Patent ductus arteriosus in preterm infants: is early transcatheter closure a paradigm shift?. J Perinatol 39, 1449–1461 (2019). https://doi.org/10.1038/s41372-019-0506-7
- Fraisse A, Bautusta-Rodriguez C et al. Transcatheter closure of Patent Ductus Arteriosus in Infants with weight under 1,500 grams. Frontiers in Paediatrics. 2020 September (8). doi: 10.3389/fped.2020.558256
- Martini S et al. To Feed or Not to Feed: A critical overview of enteral feeding management and gastrointestinal complications in preterm neonates with patent ductus arteriosus. Nutrients 2020, 12,83
Implications of race, equality & other diversity duties for this document
This guideline must be implemented fairly and without prejudice whether on the grounds of race, gender, sexual orientation or religion.