Neurological Section
The information on this page is intended for health professionals reference and use only. If you are a parent or family member looking for information about treatment, please visit unit information.
Contents
1.0 Aim of Guideline
2.0 Scope of Guideline
3.0 Guideline Summary
4.0 Guideline Framework
4.1 Introduction
5.0 Management
5.1 Antenatal Management
5.2 Intrapartum Management
5.3 Clinical / Postpartum Management
5.4 Non Pharmacological Management
5.5 Pharmacological Management
6.0 Discharge
Version Control
Appendices
Appendix A Neonatal Drug Withdrawal Score Chart
Appendix B Flow Chart for Pharmacological Treatment of NAS
Appendix C Thames Valley Morphine Treatment Flowchart
Appendix D Dosage regimes for methadone, clonidine and phenobarbitone
1.0 Aim of Guideline
The purpose of this guideline is to ensure that babies born to mothers who have drug addictions, or who are prescribed medication known to potentially cause withdrawal in neonates, are identified and managed appropriately without unnecessary removal from their mother.
2.0 Scope of Guideline
The guideline applies to all neonates, who are born in neonatal units and maternity units covered byThames Valley and Wessex Neonatal Operational Delivery Network whose mothers have been known to take illicit or prescribed drugs with potential to cause neonatal abstinence syndrome. This includes the following hospitals:

3.0 Guideline Summary
The Thames Valley Network guideline for Neonatal Drug Withdrawal, first published in 2011 has been updated and amalgamated with the Royal Berkshire Hospital Neonatal Abstinence Syndrome Guideline GL384, published in June 2015. In addition, further information regarding mothers prescribed Selective Serotonin Reuptake Inhibitors (SSRI’s), Serotonin Norepinephrine Reuptake Inhibitors (SNRI’s) and Tricyclic Antidepressants (TCA’s) and approach to management has been included.
List of abbreviations:
CNS: Central nervous system
IUGR: Intrauterine growth restriction
HIV: Human immunodeficiency virus
NAS: Neonatal abstinence syndrome
PPHN: Persistent pulmonary hypertension of the newborn
SNRI’s: Serotonin norepinephrine re-uptake inhibitors
SSRI’s: Selective serotonin re-uptake inhibitors
TCA’s: Tricyclic antidepressants
4.0 Guideline Framework
This guideline provides guidance on the management of infants born to mothers with drug addictions and mothers on prescribed medications known to cause potential withdrawal in neonates.
It is designed for use in the Maternity Unit and on the Special Care Baby Unit.
4.1 Introduction
Infants exposed to certain drugs during pregnancy may become physically dependent on them and, after birth, suffer withdrawal symptoms, termed the neonatal abstinence syndrome (NAS).
Drugs which may cause withdrawal are:
Opiates – e.g. codeine, diamorphine (heroin), methadone, fentanyl, buprenorphine, tramadol
Benzodiazepines – e.g. diazepam, temazepam, clonazepam
Barbiturates – e.g. phenobarbital Amphetamines
SSRI’s – e.g. Sertraline, Citalopram, Fluoxetine, Venlafaxine
Antipsychotic medications e.g. Quetiapine
Drugs which may cause other health concerns in the infant:
Cannabis – growth restriction, long term neuro-behavioural problems
Cocaine – vasoconstrictive effects on developing brain which may lead to neurological abnormalities
Alcohol – fetal alcohol syndrome
Drug use during pregnancy can also be associated with:
Premature labour, placental abruption, stillbirth, neonatal death (especially with cocaine abuse)
Birth defects:
- cleft lip / palate (heroin/opiates)
- Underdeveloped limbs (cocaine)
Intrauterine growth restriction (IUGR)
Meconium staining of liquor
Delayed onset of respirations / respiratory depression
Longer term problems include sudden infant death syndrome, neurodevelopmental delay, behaviour and social problems.
However, unless there are other indications it is not necessary for a paediatrician to be called routinely to attend these deliveries.
Clinical Features of Neonatal Abstinence
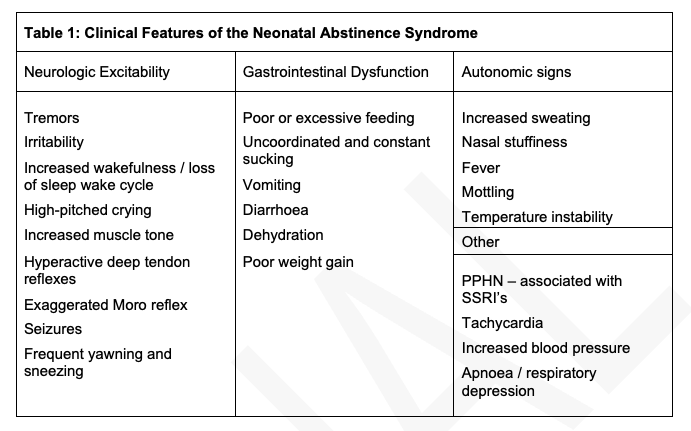
Differential Diagnosis:
Sepsis, Birth asphyxia, Hypocalcaemia, Hypoglycaemia, CNS bleeds, hyperthyroidism, hyperviscosity, milk intolerance – these must be considered when evaluating a baby for possible withdrawal.
Onset of symptoms:
This will vary depending on type of drug taken, amount of drug taken, how recently drugs were taken, use of multiple drugs simultaneously and maternal physiology.
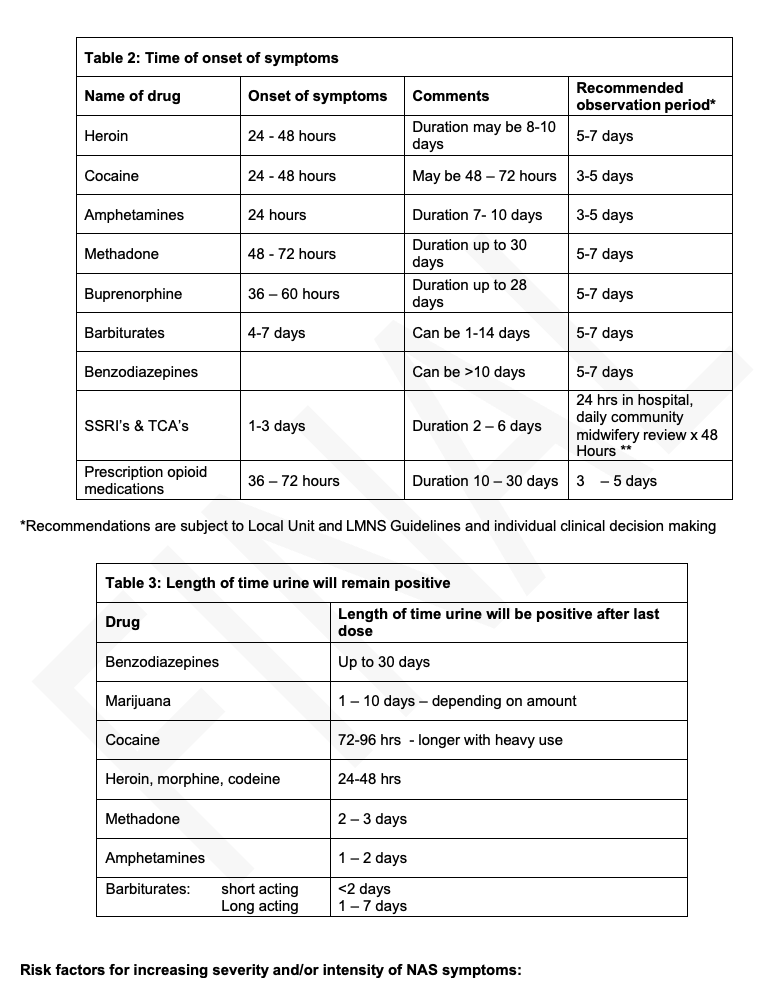
Definite:
- Term
- Good birth weight
- Polydrug use / abuse
- Combination with benzodiazepines
- Delayed drug metabolism
Probable:
- Male gender
- Maternal smoking
- Maternal methadone use
- Combination with SSRI’s/SNRI’s
Specific drugs:
Opioids:
If more than 1 week between last ingestion and birth, incidence of NAS is relatively low. Incidence and severity of NAS is increased in methadone compared with buprenorphine or heroin. Withdrawal from opioids can be severe and prolonged with subacute signs persisting for up to six months. In the acute phase, seizures have occurred in 2 – 11% of cases of NAS. Seizures are also associated with barbiturates, alcohol and sedative hypnotic withdrawal. There have been a few cases described in the literature of NAS in babies born to mothers who were prescribed codeine in late pregnancy for pain relief. Mothers prescribed codeine in late pregnancy should be warned of this possibility.
Cocaine: (Elke H. Roland et al. Paediatric Neurosciences 2989; 15:88-94)
Cocaine use is increasing in pregnant women. There is no clearly defined abstinence syndrome. Clinical features are similar to narcotic withdrawal. Withdrawal score is higher with both cocaine and heroin. There is an increased risk of hypoxic-ischaemic cerebral injury. Cerebral infarctions and intracranial haemorrhage including subarachnoid bleeds have been reported. Blood pressure measurement must be done prior to discharge. A cranial ultrasound scan should be offered but may not detect abnormalities. Cocaine is teratogenic, therefore examine carefully for congenital anomalies (gastroschisis, genitourinary, gut atresias, limb reduction defects). Abnormal visual fixation and ocular abnormalities (uncertain clinical significance) can also be found. If there are clinical concerns – obtain an ophthalmology opinion.
Selective Serotonin Reuptake Inhibitors (SSRI) & Tricyclic Antidepressants (TCA’s):
SSRI’s and TCA’s are the most commonly used antidepressants in pregnancy and are generally considered to be safe (non-teratogenic) except for a possible relationship between paroxetine and cardiac defects. However, there is a slightly increased risk of developing PPHN in babies of mothers on SSRI’s. Whilst babies on SSRI’s and SNRI’s are also at increased risk of developing toxicity or withdrawal symptoms if exposed in the third trimester, these are generally mild and very seldom require intervention. Symptoms are mainly CNS, gastro-intestinal, respiratory and autonomic. TCA’s can produce withdrawal symptoms including irritability, agitation and seizures but there is no association with developmental delay.
- Do pre- and postductal saturations within 24 hours of birth to exclude PPHN prior to discharge
- Consider observing babies in hospital for 12 – 24 hours. Babies should be observed at regular intervals (ideally daily by a suitably trained health professional) for the first 72 hours for signs of withdrawal. If no community midwifery support available, consider observing in hospital for 72 hours.
- It is not contra-indicated to breastfeed.
- If baby develops symptoms, readmit if already discharged home and, additionally, need to monitor for hypoglycaemia.
5.0 Management
5.1 Antenatal Management
- Check maternal viral serology including Hep B, Hep C, HIV
- Refer to the local rehabilitation group and to Social Services – if required
- Refer to the local Substance Misuse Liaison Midwife
- Discussion between Senior Paediatrician, Substance Abuse Liaison Midwife and Social Services +/- Rehabilitation team to formulate management plan. A Prebirth Case Conference may be necessary in some cases.
- Regular review of management plan with the above team
- Plan of action to be documented in pending birth cases file on Special Care Baby Unit as per local hospital arrangements
- Social worker/midwife to ensure that mother is aware of plan
5.2 Intrapartum Management
At delivery, avoid use of Naloxone as it may precipitate sudden withdrawal and possibly seizures (these seizures are best treated with IV morphine rather than phenobarbitone).
Ensure that Social Services are aware of the birth.
Clinical / Postpartum Management
History required from mother:
- The type of drug(s) taken (multiple drug use is very common, please also ask about prescription and over the counter preparations)
- Frequency of use for each drug
- Duration of maternal drug use
- Most recent drug use prior to delivery
- Any detoxification programmes
- Additional information may be available from the midwives or social worker
- Review any antenatal birth plans already made – check Pending Deliveries folders
Initial postnatal management:
- Parents should be involved in all care planning and delivery of care to their baby and also given the opportunity to discuss care and concerns with staff.
- The baby may stay with the mother for the immediate postnatal period unless any other medical condition is apparent. If available, admit mother and baby to a Transitional Care Ward / Unit in order to provide closer supervision of babies for withdrawal and also assessment of maternal parenting skills. A daily Paediatric review is necessary.
- Urine for toxicology must be obtained from the baby and sent to the laboratory with a chain of custody form following consent given from mother. If consent from the mother is withheld, discuss with senior paediatrician with a view to involving Social Services especially if mother is already known to them. Ensure you are aware of all prescribed medication recently given to mother before interpreting the results. If available, meconium can also be sent for toxicology
- The baby may remain under the management of the midwife on delivery suite / postnatal ward if required, unless the midwife has particular concerns about the baby’s withdrawal state.
- The baby should receive routine postnatal ward observations and if there are concerns about symptoms, the status of withdrawal should be assessed using the Neonatal Drug Withdrawal Score Chart (Appendix A)
- Should baby require treatment for NAS this should ideally take place on a transitional care ward setting with aim of keeping the baby with mother as this has been shown to shorten the treatment duration and length of stay in hospital.
- Pre- and postductal saturations should be carried out within 24 hours of birth for all babies whose mothers have been on SSRI’s.
- Consider performing a cranial ultrasound scan for all babies whose mothers have abused cocaine, particularly if heavy amounts of cocaine have been used.
- For mothers who are also HIV and/or Hepatitis B/C positive, follow local guidelines in addition to this one for management of these infants.
- For babies who are to become looked after children, Hepatitis B, C, HIV and syphilis status needs to be checked on the baby as soon as practically possible after this decision has been made and baby should be offered accelerated Hepatitis B vaccine.
- Routine Hepatitis B vaccine should be offered to both mother and baby if mother is Hepatitis B negative where narcotic abuse is identified.
- Strict patient/family confidentiality must be maintained
Breastfeeding:
Provided there are no other contraindications (e.g. HIV) – breast feeding is safe in most women on withdrawal programmes. Advise mother to take medication after feeding baby. Withdrawal may in fact be smoother in breast fed babies.
Breast feeding shortly after Cocaine ingestion can cause seizures. If mother is a heavy user and baby is having seizures breast feeding should be discouraged. A minimum 24 hour “washout” period (interval between last known cocaine ingestion and breastmilk feed / expression) is advised if mother keen to breastfeed. Milk expressed within 24 hours of cocaine ingestion must be discarded.
For most babies at risk of neonatal abstinence, breast feeding is safe and not necessarily contraindicated. However it is good practise to check the latest drugs and lactation database for current recommendations and safety profiles.
Using the Neonatal Drug Withdrawal Score Chart (see appendix A):
- Use of the scoring chart should be commenced by any member of the team looking after the baby (midwife or doctor) at the first sign of any symptoms of withdrawal (see table 1).
- If a symptom is present, score 2; If not score (There is no score of 1)
- Record one score for each section (there should only be one number in each box, either 2 or 0, so maximum possible score is 18)
- If possible document the score approximately one hour following a feed.
- Take into account behaviour appropriate for age and gestation.
- Consider symptoms present over the whole scoring period
- Document score 3-4 hourly, after 2 consecutive scores of 6 or more document the score 2 hourly
Following 2 consecutive scores of 8 or above, the Neonatal doctor must be informed and asked to review the infant. The infant will usually be admitted to the Neonatal Unit or Transitional Care if available and treatment should be introduced (see Appendices B & C).
5.3 Non Pharmacological Management
The aim of non-pharmacological measures is to keep baby as calm and quiet as possible by encouraging skin to skin, reducing infant -maternal separation and attending to baby’s needs to avoid baby developing irritability – excessive crying – insomnia cycle.
- Feeding – excessive hunger and sucking is a common symptom of withdrawal and overfeeding can occur if total daily volume is not monitored. Swaddle and allow responsive, paced feeding as much as possible without overfeeding excessively – to avoid baby becoming distressed.
- Document all feeds and discuss with the neonatal doctor and multidisciplinary feeding team if the infant is demanding more than 220mL/kg/day and cannot be soothed with swaddling and non-nutritive sucking.
- Caloric intake may need to be increased because of increased energy expenditure, vomiting and / or loose stools. Therefore, close weight monitoring is required.
- These babies are extremely sensitive to external stimuli. The baby will be fractious and distressed when over stimulated and withdrawal symptoms can increase in severity with environmental and/or physical stimulation. AHP referral should be considered where access to these services exist.
- It is possible to reduce these babies’ symptoms of withdrawal with a reduction in environmental and physical stimuli such as a dark quiet environment, avoiding auto-stimulation with, for example, swaddling, use of comforting techniques e.g., swaying, rocking, and responding early to infant cues.
- Nursing care for these babies must be individualised with an awareness of developmental aspects of care. The babies may be full term and remain on the Neonatal Unit for some weeks. Their gestation and post-natal age must be considered when planning care, observing behaviour, signs of withdrawal and responses to treatment.
5.4 Pharmacological Management
Optimal pharmacological treatment for NAS has not been established. Many drugs have been used to treat NAS. However, few randomised controlled trials have compared the efficacy of the various pharmacological agents. Once medication is commenced, hospital stay is invariably prolonged.
Opioids are currently considered first line treatment, however new studies suggest that buprenorphine (Ref4) or methadone (Ref5) may be preferable as first line treatment especially for opioid drug withdrawal. For doses see Appendix(D)
Phenobarbitone is considered second line treatment and has been effective in treating opioid withdrawal seizures especially in addition to morphine. If maximum morphine dose is not controlling symptoms, consider adding phenobarbitone as second line agent. Clonidine has been shown to be an effective and safe alternative second line treatment for NAS symptoms refractory to opioid therapy. It can also be used in combination with morphine. However, tolerance to clonidine can develop quickly, hence weaning from clonidine needs to be expedited as well.
For infants exposed to polydrug abuse, consider a combination of phenobarbitone and morphine as this has been shown to shorten length of stay compared to morphine alone.
Other drugs which could be considered are: buprenorphine and methadone–especially if the mother was using these drugs during pregnancy.
For NAS secondary to non-opiate drug use (including SSRI’s, SNRI’s, TCA’s, cocaine), phenobarbitone is considered first line treatment.
Naloxone at delivery is to be avoided for treating respiratory depression as it may precipitate abrupt withdrawal symptoms and induce refractory seizures in the neonate.
Seizures – Loading dose of Phenobarbitone in addition to Morphine as above. Phenobarbitone alone may not be effective in treating seizures caused by narcotic withdrawal at withdrawal treatment doses.
If there are seizures then the infant should be admitted to the Neonatal Unit urgently and treated as per local seizure policy – but see above points as well.
5.5.1 Indications for pharmacological treatment:
- Supportive / non-pharmacological measures fail to control baby’s symptoms
- Withdrawal scores remain high
- Seizures or other serious symptoms / signs of NAS
- Withdrawal is associated with dehydration due to diarrhoea and / or vomiting
See Appendices B & C for initiating and weaning medication.
6.0 Discharge:
Discharge planning is undertaken according to normal unit procedure, but in addition consideration must be given to the following:
- The family may have been known to supportive agencies and Social Services antenatally in which case a discharge plan should have been made and documented on the maternal notes and/or prenatal birth plan.
- Where disclosure of drug use has not been made during pregnancy or where the mother and/or family is not known to supportive agencies, a Needs Assessment is required and appropriate referrals should be made, including to Social Services. This may be undertaken by medical or nursing/midwifery personnel with parental consent.
- Where concerns have been raised antenatally about the mother’s capacity to meet the needs of her baby in the light of her drug misuse a pre-birth planning meeting/case conference has usually taken place and decisions made which may impact on the discharge plan.
- Following delivery, liaison with the allocated Social Worker is necessary. If the baby requires a prolonged hospital stay in the Neonatal Unit for management of withdrawal, a discharge planning meeting is arranged with Social Services, Community Drug Team Worker, Health Professionals and the family to facilitate co-ordination of an appropriate discharge plan.
- For mothers on prescribed medication such as benzodiazepines, SSRI’s, referral to Social Services is not required unless other concerns have been identified. However, close liaison with mother’s midwife, usually a midwife lead for mental health, is advised. Similarly, mothers who used cannabis early in pregnancy but who gave up following discovery / diagnosis of pregnancy do not necessarily require Social Services involvement unless other concerns have been identified.
- Out Patient follow-up will be arranged where best suits the baby’s needs during the discharge planning process. However, long term follow-up is not often necessary.
- If no symptoms of withdrawal – discharge to primary care,
- If baby does show signs of withdrawal – observe in hospital till resolving / drug treatment is commenced.
- Babies needing treatment can be discharged when on a weaning regime (usually after about 2 weeks of hospital treatment) depending on individual circumstances. Individual hospital policy may vary.
- If discharging on treatment – Dispense only 1 week’s prescription at a time. Paediatric community Nurse should be involved in addition to Health Visitor. Follow-up reviews to be carried out as per local guidelines.
Lead authors
Dr Zuzanna Gawlowski, Consultant Paediatrician, MKUH
Dr Mushtaq Soomro, SAS Doctor Paediatrics, MKUH
Denise Falcus, Advanced Neonatal Nurse Practitioner
Dr Rekha Sanghavi, Consultant Paediatrician
Approved by
Thames Valley & Wessex Neonatal ODN Governance Group
Approved on
Renew date
January 2026
Full guide
Related documents
References
Cleary BJ, DonnellyJ, Srawbridge J et al. Methadone doses and neonatal abstinence syndrome-systematic review and meta- analysis. Addiction 2010;105:2071-84
Dryden C, YoungD,, Hepburn M, Mactier H. Maternal methadone use in pregnancy: factors associated with thedevelopment of neonatal abstinence syndrome and implications for healthcare resources. BJOG 2009; 116:665-71
Hall ES, Wexelblatt SL, CrowleyM et al. A Multicentre cohort study of treatment and hospital outcomes in neonatal abstinence syndrome. Pediatr. 2014 Aug;134:527-34
Nair V, Soraisham AS, Akierman A. Neonatal withdrawal syndrome due to maternal codeine use. Pediatr ChildHealth. 2012;17(5):e40-41
Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev 2010:CD002059
Seligman NS, Almario CV, Hayes EJ et al. Relationship between maternal methadone dose at delivery and neonatal abstinence syndrome. J Pediatr 2010:157-428
Wiles JR, Isemann B, Ward LP et al. Current management of neonatal abstinence syndrome secondary to intrauterine opioid exposure. J Pediatr 2014: 165-44
Elke H. Roland et al. Paediatric Neurosciences 1989; 15:88-94
Hudak ML, Tan RC. Neonatal Drug Withdrawal. Pediatrics 2012;129(2): e540 – e560.
Jeferries AL et al. Selective Serotonin Re-uptake Inhibitors in pregnancy and infant outcomes. Canadian Pediatric Society position Statement. Posted 01/11/2011. Reaffirmed 01/02 2016. Abridged version published in Paediatric Child Health 2011; 16(9): 562.
Forsberg L et al. Neonatal Adaptation in Infants prenatally exposed to antidepressants – Clinical Monitoring using Neonatal Abstinence Score. PLOS ONE 2014;9(11): e111327
MHRA Drug Safety Update May 2010 Vol 3 Issue 10:2. SSRI’s and SNRI’s: Risk of Persistent Pulmonary Hypertension of the Newborn.
Grigordiadis S et al. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ 2014; 348: f6932.
Hamdan A H. Neonatal Abstinence Syndrome http://emedicine.medscape.com/article/978763-overview
Updated Nov 27, 2016.
Kocherlakota P. Neonatal Abstinence Syndrome. Pediatrics 2014; 134: e547-e56
1. Holland J. et al Neonatal Venlafaxine discontinuation Syndrome :A mini review. Pediatric Neurology 2016
2. British National Formulary for Children page 243
3 .MacMillan K.D.L. et al Association Of Rooming –in –with outcomes for Neonatal Abstinence Syndrome. JAMA Pediatrics April 2018
4 .Lacaze-Masmontiel T, O’Flaherty P. Managing Infants born to mothers who have used opioids during pregnancy. Pediatr Child Health 2020
5. Jansson L.M. Neonatal abstinence syndrome UP TO DATE Sep 202
6. Kraft WK, Adeniyi-Jones SC, Chervoneva I. et al. Burenorphine for the Treatment of the Neonatal Abstinence Syndrome. N Eng J Med. 2017 Jun 15: 376(24); 2341-2348
Implications of race, equality & other diversity duties for this document
This guideline must be implemented fairly and without